
Neuroscience: Shooting pain
for NatureOutside neurology and his family, Sean Mackey doesn’t have many hobbies. The one exception is his monstrous flat-screen television and large film collection. Driving to Stanford, California, on the day I am to visit Mackey’s lab for testing, I am reminded of a scene from his favourite movie, The Princess Bride. In the film, the villain, Count Rugen, straps the hero Westley into a sinister apparatus and confesses a “deep and abiding interest in pain”. Then he tortures the hero in the name of science.
It turns out that this is not far from what is in store for me.
Mackey heads the Pain Management Center at Stanford School of Medicine where, as part of his research on ways to relieve pain, he routinely inflicts it. Widely seen as one of the field’s rising stars, Mackey is part of a movement to upend the way scientists look at pain, drawing the focus away from the nerves that sense it, towards the brain that processes it. His primary tool is functional magnetic resonance imaging (fMRI), which can create images of the brain responding as the body is hurting. The trick now — and one focus of Mackey’s work — is to understand whether a person can consciously change the way the brain processes and perceives pain. That’s where I come in. The plan is to put me inside the fMRI scanner, apply burning heat, and see whether I can train myself to regulate my pain.
As part of his studies, Mackey has found himself struggling with a question facing the fMRI field as a whole: when is the technique ready for use outside the lab? One of his former colleagues has started a company that plans to offer patients the fMRI ‘feedback’ pain-control technique that Mackey was involved in developing. But Mackey has distanced himself from the company in these early stages, based on what he has observed elsewhere. “I’ve seen too many treatments that are the next latest and greatest thing out there that people get really excited about. Everybody gets on board and initially the results are fantastic. And then as time goes by we start to see that the results are not as good as initially proposed,” he says. “And then you find out that it doesn’t work at all.”
Walking into his office near the Stanford Hospital, Mackey is more reminiscent of a corporate executive than a brain researcher. He is a cheery, focused ball of energy, with a quick smile and a firm, reassuring handshake. He regularly wears a suit in the lab. He says it fights the stereotype that all anaesthesiologists — he trained as one — wear “pyjamas” to work.
Destructive force
According to Mackey, the suit also tells patients that he takes his work seriously. They may be recovering from knee surgery, they may be wounded veterans or they may have a rare neurological condition that can cause excruciating full-body pain. According to the International Association for the Study of Pain, one in five people endures moderate to severe chronic pain. “I have seen it take people who are otherwise normal and turn their lives upside down and absolutely destroy them,” says Mackey. Or as his colleague Ian Carroll, another Stanford anaesthesiologist, puts it: “It’s like the black hole of the brain. It dominates it and forces everything to spin around it.”
“I have seen pain turn people’s lives upside down and absolutely destroy them.”
The experience of pain typically starts in receptors near the skin called nociceptors that transmit information through axon fibres to neurons in the spine, then to the brain. Until the 1990s, pain research focused mostly on nociceptors as well as neurons near the spinal cord. Pain experts would treat a backache, say, directly on the back. If they addressed the brain, it might have been with opioids, whose mechanisms were somewhat mysterious.
The arrival of the fMRI scanner changed that. The technique is an indirect measure of neural activity in the brain: as a region activates, it consumes oxygen, and neurologists use fMRI to track fresh oxygenated blood surging in to replace the old. “Imaging is now a huge part of the field,” says Allan Basbaum, editor of the journal Pain. Along with behavioural studies, imaging has helped to build the view that pain involves many brain areas and that chronic pain may cause long-term changes to the morphology or function of some of these regions.
As a technique, fMRI has its share of detractors. Interpreting the image relies on complicated analyses that link blood movement to a given task performed by the subject, and many neuroscience studies have come under fire for poor data analyses or interpretation. Although Mackey is a devoted user of fMRI, he is also an occasional critic. He has testified against the use of the technique in several court cases where defendants wanted to use fMRI images to prove they were telling the truth.
“This is an interesting time in the use of this tool,” says Mackey. “You are now seeing that the application has gotten easier and easier. It’s still not quite like ordering a McDonald’s Happy Meal, where you put somebody into the scanner, press a button, and the brain pictures come out, but we are probably going to get there.”
“You can’t yet press a button and the brain pictures come out, but we are probably going to get there.”
It may seem odd to base so much of one’s scientific life on a single tool and then lobby against its application. But Mackey has a different background from many pain researchers. In addition to the MD in anaesthesiology, he has a PhD in electrical and computer engineering from the University of Arizona. He chose to work in neurology — he calls it an uncharted “Wild West” — because he could treat patients and flex his engineering muscles. This training, colleagues say, gives him a valuable understanding of the biology of pain, the complex instrumentation for measuring it and the limitations of that technology.
Personal limits
Mackey thinks that pain could be a useful proving ground for fMRI. Unlike hard-to-define cognitive or emotional states — say, deception, jealousy or anger — pain can be elicited in a controlled way at specific levels, is highly repeatable and leads to a common response: it hurts. The intensity of the pain experienced varies from person to person, but can be ranked on a scale.
 Nature ‘s reporter prepares for pain.A. M. SAWYER
Nature ‘s reporter prepares for pain.A. M. SAWYERThe previous day, I had gone to have my own pain threshold tested. A friendly doctor took me into a small room and strapped to my arm a ‘noxious thermal stimulus’, more accurately a ‘hot metal pad that hurts’. She added a pepper-based cream that made my skin sensitive to heat. Then she slowly worked me up to a pain level that I ranked as seven out of ten. A seven is considered the worst pain a person can tolerate without moving, and everyone will reach their seven at a different level of heat.
In the fMRI machine, Mackey planned to use the same burning metal plate to take me directly to seven and leave me there. I would be practising a technique that grew out of a collaboration between researchers at Stanford and Christopher deCharms, a visiting researcher from the Massachusetts Institute of Technology (MIT) in Cambridge. The team showed people a changing image — a line graph or a picture of a fire — representing the real-time fMRI signal in their anterior cingulate cortex, a region commonly studied in relation to pain. They showed that people could learn to manipulate the fMRI signal and their perception of pain intensity through visualization exercises, such as ‘turning down’ pain like a radio dial. The team reported in Proceedings of the National Academy of Sciences (PNAS) that patients with chronic pain reported on average a 64% reduction in pain on one scale1. The study showed that fMRI could be used not only as a diagnostic, but as the means to therapy itself, and that people can exert conscious control over specific brain regions much as it is known that some people can consciously alter their heart rate.
In some of his other work, Mackey’s laboratory has used fMRI to explore these connections between pain processing and cognitive processes. Fear of pain, for example, can increase the pain itself, and Mackey’s group studied some of the brain regions involved in this anticipation2. In another study3 he showed that watching someone else in pain activates brain areas that are fairly distinct from those active during one’s own pain. And in unpublished work he has found that romantic love can lessen the experience of pain. Mackey says these connections demonstrate how strong an influence conscious thought may have over pain processing.
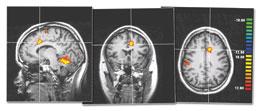 Studies suggest that people can learn to control pain using feedback to show them their brain activity.REF. 1
Studies suggest that people can learn to control pain using feedback to show them their brain activity.REF. 1But can this conscious control be put to use? Inside the coffin-sized tube of the fMRI machine, spasms in my back from its powerful magnet distract from the burning plate strapped again to my arm. On a screen above, I can see a squiggly line that represents the activity in a part of my anterior cingulate cortex. Mackey asks that I envision the heat as alternately searing and soothing. The aim is to master control of the line so that it (and thus my pain) goes up and down. As I switch between these visions the line on the screen twitches up and down.
It is surprisingly difficult. Willpower and meditation have little effect, and after two hours it is increasingly hard to make the stubborn little line move at all. The PNAS study suggests that it would probably take several sessions before I could be deft enough to reduce other pains in my body.
Commercial approach
DeCharms, first author of the PNAS paper, started a company called Omneuron in Menlo Park, California, to offer real-time fMRI sessions as a form of therapy. The company has already garnered a fair amount of attention and is funded by the US National Institutes of Health (NIH) in Bethesda, Maryland, to conduct a phase II clinical trial in people with pain conditions such as neuralgia, fibromyalgia or migraines. As a control, some participants will see feedback from a previous participant’s brain. Omneuron is also investigating feedback fMRI to help addicts combat their cravings. DeCharms said in a short interview that feedback fMRI may someday be a valuable tool to ease chronic pain. Mackey has little to say directly about Omneuron, beyond cautiously wishing them good fortune. He adds that Stanford currently has no connections with the company’s trials.
“There is something different about the balance of goals that a company has versus a nominally disinterested science project,” says John Gabrieli, deCharms’ former supervisor at Stanford and now at MIT. “I think that is partially why Mackey and deCharms parted ways. I think they couldn’t find that balance between them.”
Gary Glover, who directs Stanford’s radiological-sciences lab, was a co-author on the PNAS paper and is more openly sceptical of attempts to commercialize feedback fMRI. He points out that thePNAS study determined that the participants benefited on average. This does not necessarily mean that the technique would work for any particular individual though. And customizing the feedback fMRI is generally more difficult than customizing the dose of a drug, because each person might be helped best by targeting a different brain region with a different mental exercise.
Glover also worries that with such a new technique, no one has any idea if it carries side effects: perhaps after a while the pain will worsen, or perhaps the therapy could stop patients feeling useful pain in other situations. “There are ethical questions when you are in the middle of someone’s cognition,” says Glover. Linda Porter, who oversees the NIH programme funding Omneuron’s trial, says the obstacles are surmountable and that, compared with drug trials, the minimal risk of side effects is one of the appealing aspects of feedback fMRI.
“There are ethical questions when you are in the middle of someone’s cognition.”
Mackey is split on the issue of fMRI therapy. As a clinician he thinks that research should be guided by patients’ needs and that new technology should be released as soon as possible. As a researcher, he stresses that it is too early for clinical use. Pain therapies are highly subject to placebo effects, which often boost initial results and then whither away during later trials. The therapy is dependent on clinicians’ ability to locate the correct part of the brain to show a patient. Much of the research so far has targeted single regions of the brain, yet it is possible that multiple regions are involved.
Mackey admits that he doesn’t know what is required to convince him fMRI therapy is ready for patients. But he is working to convince himself. His latest study is a feedback-fMRI experiment aimed not at specific brain regions, but at the connections between them. He is also experimenting with new ways to visualize the pain and, of course, ways to apply it.
It is easy to see why some are so excited about the possibilities of fMRI therapy. It could allow the vast body of research into brain imaging to have a direct outlet that benefits patients. If it works, it would be the first non-invasive, drug-free method of directly treating specific regions of the brain, with potential applications beyond pain relief, such as addiction and depression.
But it is not without its shortcomings. After two hours in the fMRI machine, I am stiff and woozy. It is likely I have more pain than when I went in. Mackey assures me that regulating pain is in no way attached to intelligence, which seems like a polite way of saying I did not do very well. Afterwards, we sit on a shady bench and talk about the potential of the mind to regulate itself.
ADVERTISEMENT
Still a bundle of energy, Mackey enthuses about the future of pain treatment in general, calling current technology “the dark ages”. He has no doubt that self-directed treatment such as feedback fMRI is the future, perhaps done alongside drug prescriptions and physical therapy.
He adds that he is looking for volunteers for his latest round of experiments.
I stay quiet, and rub my arm a little.